Duoning founded
Company
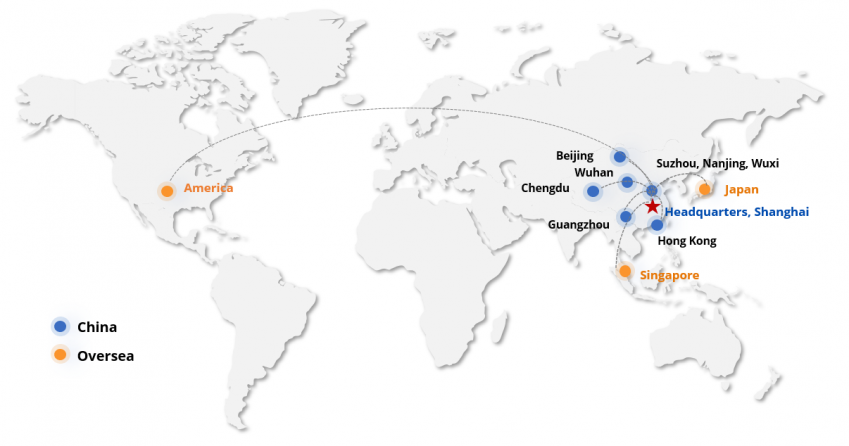
Duoning Biotechnology Group
Duoning is a leading one-stop bioprocess solutions provider in China, dedicated to providing comprehensive solutions for biological products from R&D to commercialization, including reagents, consumables, instruments, equipment and services. We operate two main business lines, bioprocess solutions and laboratory products and services. Our one-stop Bioprocess system will support customers to achieve efficient, stable, high-quality and cost-controllable drug R&D and manufacturing processes.
Duoning has more than 700 employees, 260 patents and patent applications, and 11 production facilities. We are committed to providing upscale and affordable products and services to pharmaceutical companies, CROs/CDMOs and research institutions with our technically specialized team, strong research and manufacturing capabilities and international presence. Currently, we offer more than 220 products and 140 services in the areas of antibodies, cell and gene therapy (CGT), vaccines, etc.
Duoning Mission
To serve life sciences, enhance human health!Duoning Vision
To be a leader in the global life sciences industry!Duoning Values
Focus on Innovation · Focus on Quality · Focus on Professional · Win-Win CooperationProvide One-Stop Service for Customers
Duoning has built culture media development and manufacturing platform with completely independent intellectual property rights. Based on cell metabolism and physiological research, the R&D team has developed a series of serum-free media for the biopharmaceutical industry based on the needs of the biotechnology industry. With the vigorous development of China's biopharmaceutical industry, the company focuses on improving the research and development and production efficiency of biopharmaceuticals, continuously improving the efficiency of cell culture, and accelerating the research and development and industrialization of biopharmaceuticals; at the same time, we are dedicated to help customers optimize cell culture processes and achieve win-win cooperation. Based on this principle, we provide customization of high-quality and unique cell culture media according to customer needs; develop and produce disposable biopharmaceutical process equipment and consumables, including bioreactors, filters, etc. We also provide professional GMP compliance testing and validation services to organizations and institutions of new drug research and development, biotechnology development, and medical devices, including biosafety testing, instrument and equipment verification, and on-site services.
Duoning always takes "providing customers with one-stop service" as its goal, and strives to become a comprehensive service provider with complete solutions for biopharmaceuticals industry.
Duoning Group










2005
Duoning founded
2007
Cell culture media development platform built
2017
Formed “Antibody Production Raw Materials and Equipment Localization Alliance”
with WuXi Biologics and Lisure Science
2018
Merged culture media business unit of Mab Works
Built dual-engine research and development sites in Shanghai and Beijing
Completed Series A financing
Introduced Ningbo Hongjia
2019
Completed A+ round financing
Introduced WuXi Biologics
Acquired Guangzhou QiZhi
Expanded our business to bioreactors
2020
Acquired Jinke Filtration
Expanded our business to filters
Acquired Lianghei Technology
Expanded our business to single-use products
Completed B round financing
Introduced Lake Bleu Capital,Sequoia China and LYFE Capital
2021
Acquired ATS
Expanded our business to nano particles preparation systems
Acquired CMALAB
Expanded our business to validation services
Completed C round financing
Introduced SCHP, GL Capital and CUAM
2022
Acquired Chubo Bio
Expanded our business to chromatography resins
Acquired RePhiLe
Expanded our business to laboratory water purification systems
Acquired Salus Bioscience
Established an international sales platform
Acquired Tchuyee
Enhanced our bioreactors
Completed Series C+ financing
Introduced CSPC Pharma, Rongchang Chuangtou, Akeso and KeyMed Biosciences
Leadership

Jerry Wang
President/Executive Director/Chief Executive Officer

Sam Sun
Executive Director/Executive Vice President/Chief Operating Officer

Leo Xie
Senior VP
11 Main GMP Manufacturing Sites

Shanghai: Shanghai Duoning culture media factory (1300m2)
Capacity:
① single batch 500L liquid media production line;
② single batch 200kg dry powder media production line;
Features:
① C+A class GMP production suite;
② Advanced production process ensures the uniformity of powder distribution.
Shanghai: Lianghei single-use products factories (8000 m2+11311 m2)
Capacity:
① Extrusion tubing, injection tubing production line; annual output 300,000 pcs;
② Liquid storage bag, mixing bag production line; annual output 300,000+1,500,000 pcs;
③ Customize manifold assemblies; annual output of 200,000 sets + 800,000 sets;
Features:
① Provide extractable & leachable test services and reports as requested;
② Comply with the production requirements of biopharmaceutical GMP suite.
Shanghai: Tchuyee bioreactor factory (2639 m2)
Features:
① Strict quality control;
② Manufacturing capability to produce products in compliance with GMP.
Shanghai: Jinke filters factory (12000 m2)
Capacity:
① Annual production of 600,000 cartridges;
② Independent brand "Jimei" cartridge;
Features:
① Class D cleanroom that meets GMP requirements;
② Focus on microfiltration for 27 years, and master the core technology of cartridge manufacturing process;
③ Complies with ISO9001:2015.
Suzhou: ATS nanometer technology factory (2400 m2)
Features:
① Obtained the CE certification.
Suzhou: Bogen chromatography resins factory (2000 m2)
Capacity:
① Annual output of nearly 35,000 liters of chromatography resin;
② Provide purification process development technical services;
Features:
① Commercial production of super-microporous chromatography resin.
Wuxi: Wuxi Duoning culture media factory (10500 m2)
Capacity:
① Non-GMP 1-8kg powder media production line;
② GMP 100-1000kg powder media production line;
③ GMP 4000L automatic liquid media production line;
Features:
① Class D GMP production suite; fully closed process avoids adventitious contamination;
② Advanced production technology ensures powder stability and uniformity.
Wuxi: Wuxi Duoning filters factory (9800 m2)
Capacity:
① Production line for full series of capsular filter;
② Production line for full series of depth filter;
③ The first phase factory produces 1,000,000 cartridges per year;
Features:
① Class C cleanroom that meets GMP requirements;
② The fully enclosed dust-free environment ensures the safety and stability of each cartridge.
Jiaxing: RephiLe laboratory water purification systems factory (8461 m2)
Features:
① Complies with ISO9001:2015.
Guangzhou: Qizhi bioreactor factory (6000 m2)
Capacity:
① Complete set of bioreactor production lines for lab, pilot and production scale;
② Provide large-scale cell culture technology and process optimization services;
Features:
① Complies with ISO9001:2015;
② Obtained the Intellectual Property Management System Certification.
Copyright © Shanghai Duoning Biotechnology Co., Ltd. All Rights Reserved Sitemap | Technical Support: 
Message-